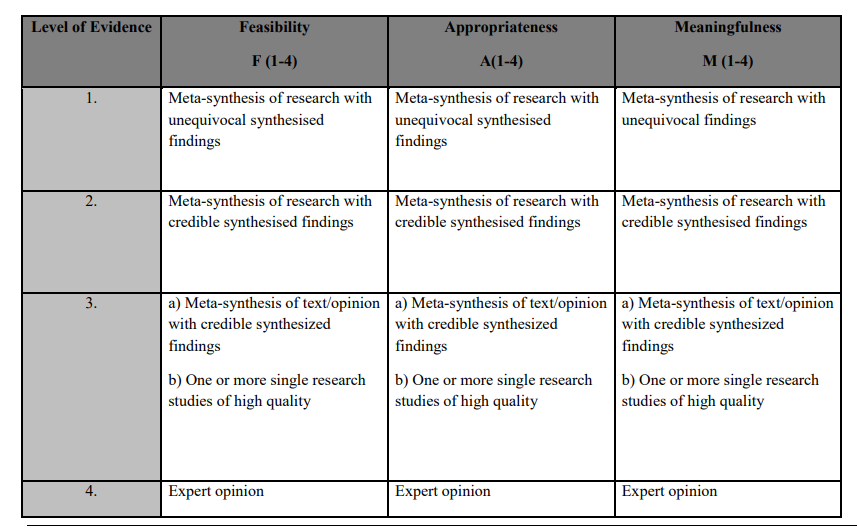
Johns Hopkins Levels of Evidence
Medical Library Literature Search Tool
 Medical Library - Literature Search Worksheet1.pdf
Medical Library - Literature Search Worksheet1.pdf
If you would like to use a tool to help organize your literature search - please click on the worksheet above and print.
Literature Search Terms

After you have formulated your PICO question, the second step is to formulate your literature search strategy using terms that correlate with your topic. You can use terms that are considered Subject Headings and their Synonyms, or you can use Keywords. A combination of these terms will yield the best search results. Limit your search by selecting Boolean Operators such as "and/or". Use the "Limits" option to select Peer Reviewed Journals or to select a date range. Below you will find a printable Literature Search Strategy Worksheet that will help you to keep track of your search history and search terms ensuring a complete review of the evidence based literature.

ClinicalKey - Clinical Key is a point-of-care clinical information service. It provides access to a unique combination of medical reference books, medical journals, drug information, and practice guidelines.
CINAHL - Cumulative Index for Nursing and Allied Health Literature - search Evidence Based Practice Nursing Literature.
Cochrane - Database of Systematic Reviews
DynaMedex - Point of Care Tool - comprehensive bullet points of treatment options, in-depth drug information, and advanced search capabilities of Micromedex
MICROMEDEX - Evidence-based clinical reference consists of a set of clinical decision support knowledge bases, including NeoFax Pediatrics, DRUGDEX, MARTINDALE, REPRORISK, POISINDEX, and RED BOOK Online.
Ovid Medline - Advanced Literature Searching access to databases such as EmCare, and Medline.
PubMed - Literature searching access that links to Sarasota's holdings in PubMed - full-text available.
STAT!Ref - Full-text searchable medical books.
TRIP Database - clinical research evidence
Natural Medicines - Clinical data on natural medicines, herbal medicines, and dietary supplements used in the Western world is compiled by pharmacists and physicians.
UpToDate - UpToDate is an evidence-based point-of-care resource authored by physicians.
Evidence Based Practice Resources
Agency for Health Care Research and Quality - AHRQ's mission is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans.
Bandolier: Independent, evidence-based health care commentaries written by Oxford UK scientists.
Clinical Trials.gov: Provides regularly updated information about federally and privately supported clinical research in human volunteers.
Institute for Clinical Systems Improvement:This independent, non-profit group maintains this site which contains links to guidelines, preventive services, summary reports about clinical outcomes.
ECRI Guidelines Trust (content from former National Guideline Clearinghouse):Evidence-based clinical practice guidelines.
U.S. Preventive Services Task Force: USPSTF conducts scientific evidence reviews of a broad range of clinical preventive health care services and develops recommendations for primary care clinicians and health systems.
Study Design Definitions
Meta-analysis
A quantitative method of combining the results of independent studies and synthesizing the results into summaries and conclusions.
Systematic Review
A review of a clearly formulated question that uses systematic and reproducible methods to identify, select and critically appraise all relevant research on any given medical subject or condition. They authors collect and analyse data from all published and unpublished material on a specific question.
Randomized Controlled Trial (RCT)
A clinical trial involving one or more new treatment strategies with at least one control treatment and one specified outcome measure for evaluating the intervention. The treatment may be a drug, device, or a new procedure.
Cohort Study
In cohort studies, groups of individuals, who are initially free of disease, are catogorized according to exposure or non-exposure to a risk factor. They are then followed over time to determine the incidence of the intended outcome of study.
Prospective cohort study
Similiar to the Cohort Study except the exposure information for the study subjects is collected at the onset of the study and new cases of disease are identified from that point forward.
Retrospective cohort study
Similiar to the Prospect cohort study except the exposure status was measured in the past and the disease identification has already begun.
Case-control Study
Studies that start by classify the subjects with and without a disease being studied. The study then retrospectively assesses the differences in exposure to the risk factors.
Cross-sectional Study
Studies in which the presence or absence of a disease are assessed in each member of a population during a particular time frame.
Evidence Based Medicine Pyramid
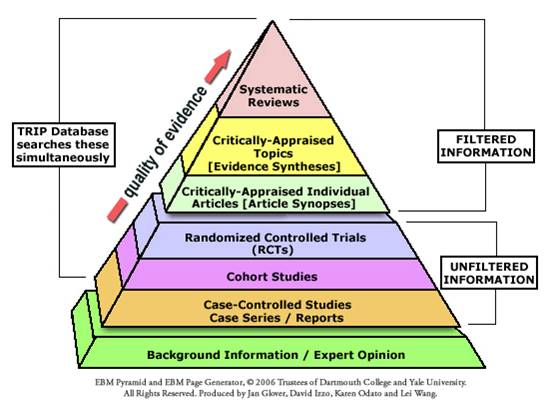
Evidence grading is a process of evidence acquisition and appraisal. The higher the grade of evidence, the more likely the study is able to predict effective outcomes.
PICO Question Type = Type of Study
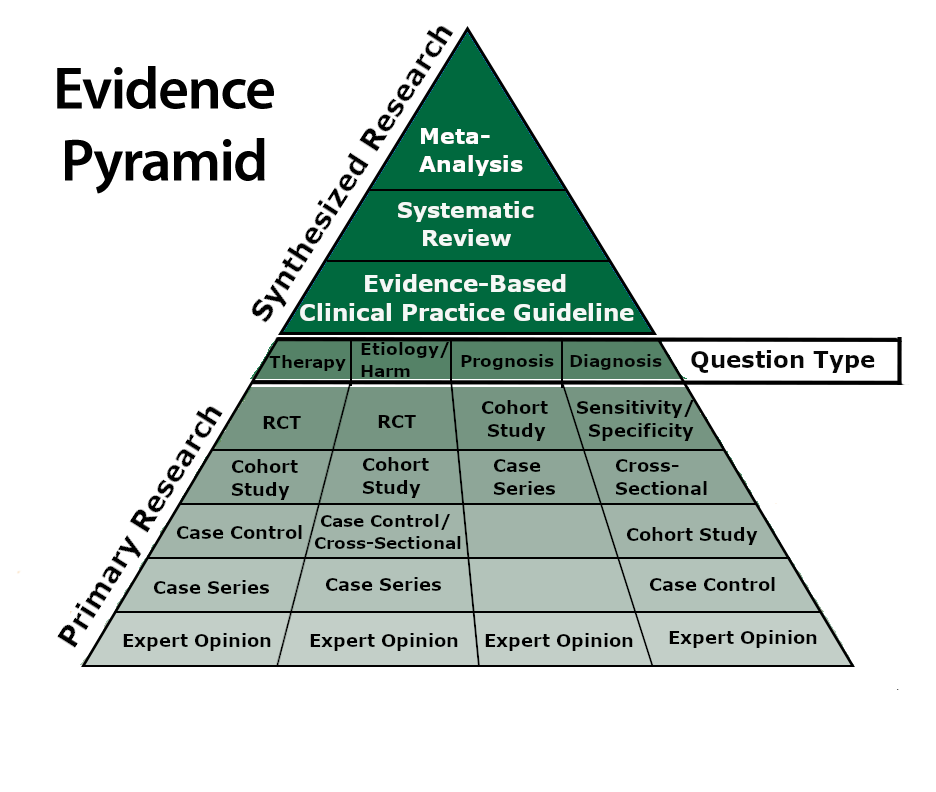
The question type can help determine the type of study one should search for within the literature. For example, if your PICO question concerns a therapy, a Randomized Controlled Study article (or a Systematic review of RCT articles) would be an ideal study type to predict effectiness of a therapy.
HEIRARCHY of Evidence
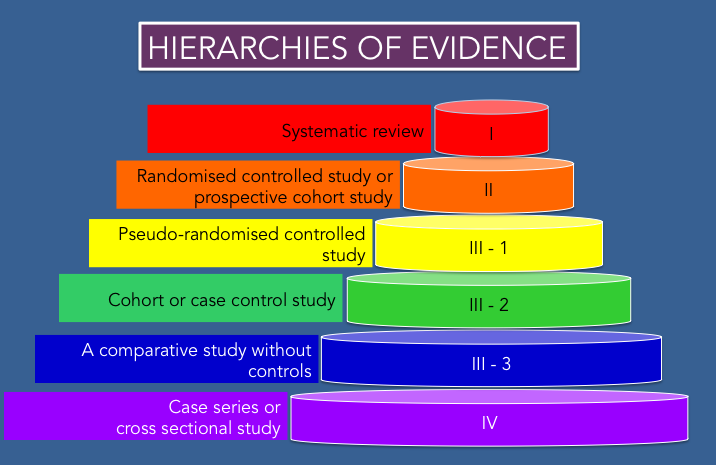
Not all evidence is created equal. This pyramid represents the quality of information associated with a study type. The most reliable forms of evidence are represented at the top of the pyramid.











